Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
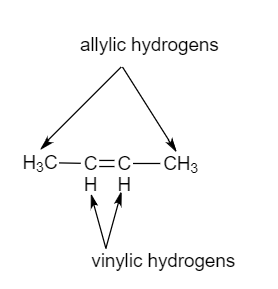
Allylic and vinylic hydrogens.
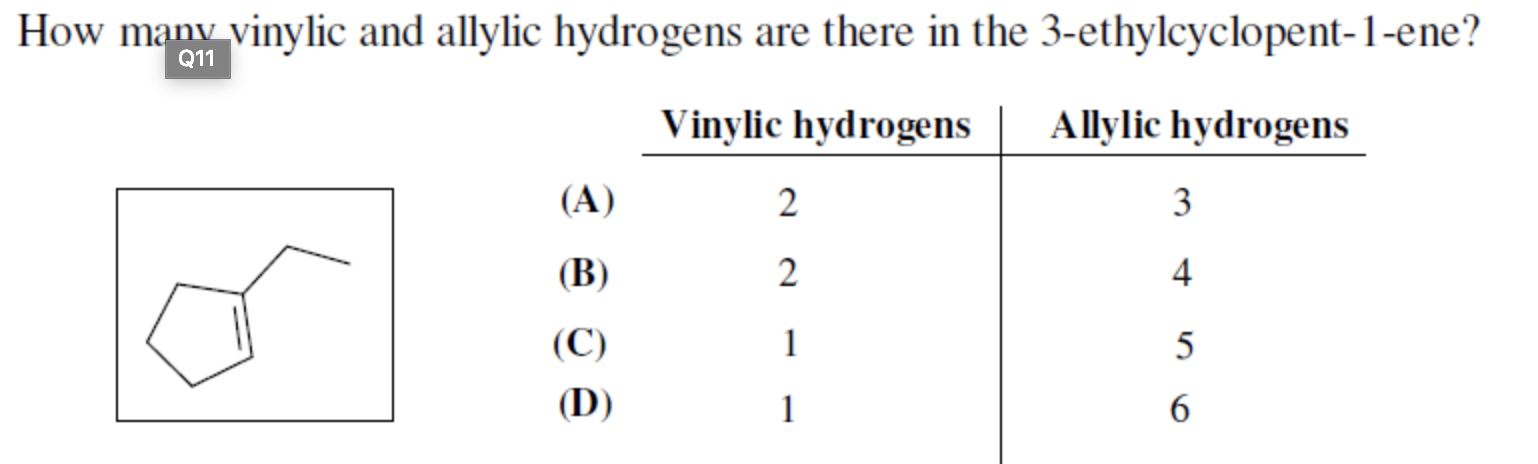
Identify the number of allylic and vinylic hydrogens in the pictured molecules.
Allyl form a stable carbocation because of the electron delocalization whereas vinylic carbocations are unstable as they lack p character.
Allylic vinylic examples organic chemistry duration.
An allylic carbocation in which an allylic carbon bears the positive charge.
The libretexts libraries are powered by mindtouch and are supported by the department of education open textbook pilot project the uc davis office of the provost the uc davis library the california state university affordable learning solutions program and merlot.
In other words it is a methylene bridge ch 2 attached to a vinyl group ch ch 2.
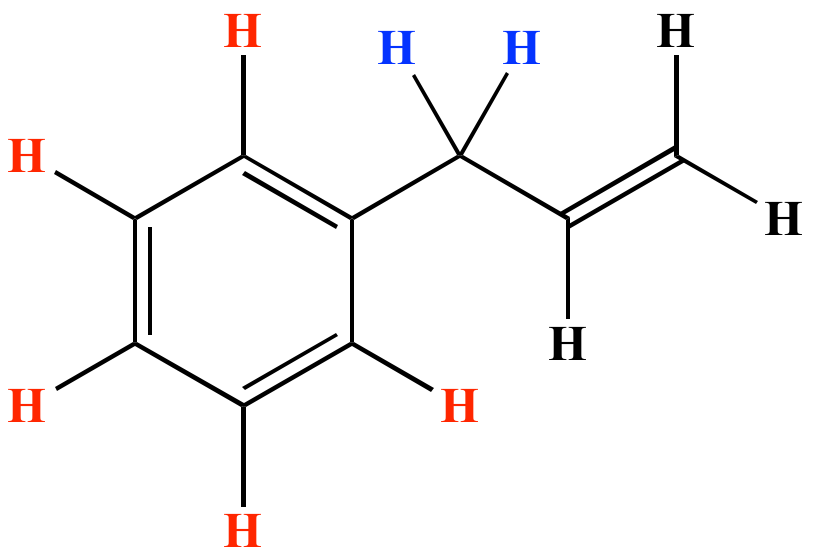
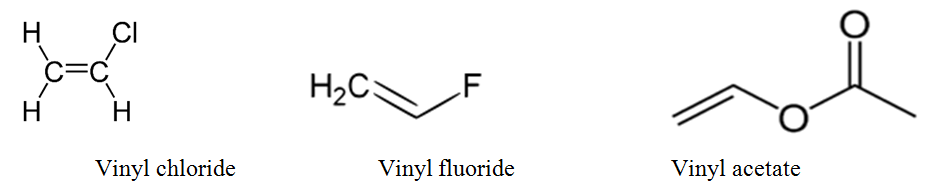
Vinyl group vinylic hydrogen vinylic carbocation allylic position benzylic position propargylic position wikipedia entry return to glossary index.
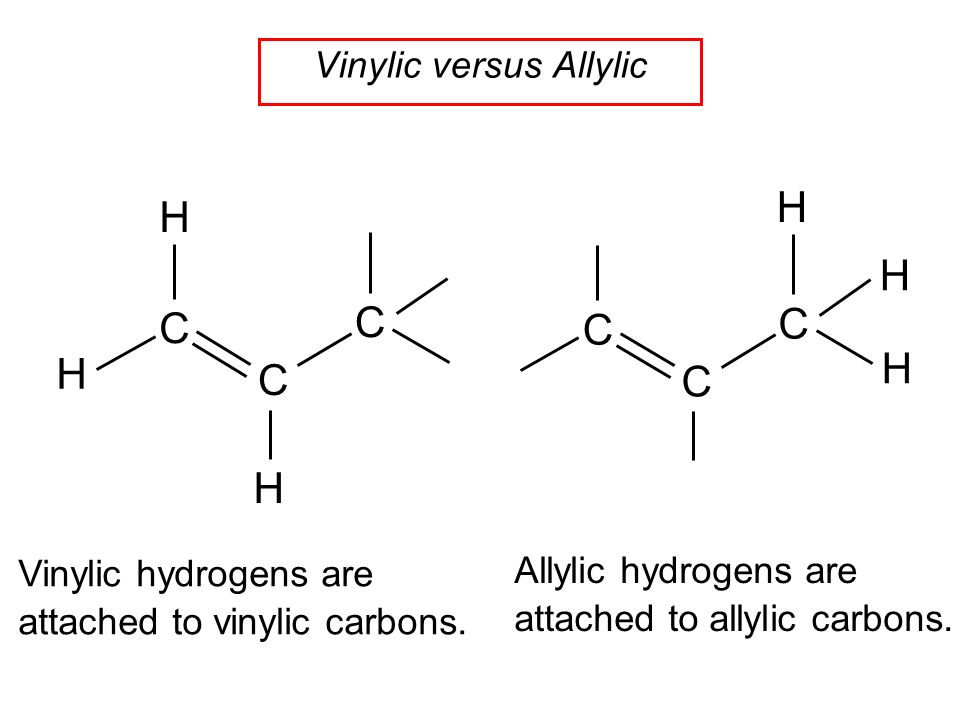
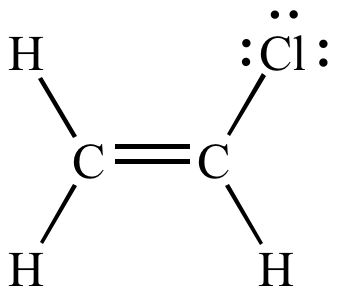
The vinylic hydrogens are shown in red.
Identify the number of allylic and vinylic hydrogens in the pictured molecules.
When one hydrogen atom is removed from the third carbon atom of a propane molecule it is equivalent to an allyl group.
A hydrogen atom bonded to an sp 2 carbon of an alkene.
An allylic hydrogen is a hydrogen atom that is bonded to an allylic carbon in an organic molecule.
The organic chemistry tutor 93 912 views.
It contains two sp 2 hybridized carbon atoms and one sp 3 hybridized carbon atom.
The key difference between allylic and vinylic carbon is that allylic carbon is the carbon.
An allylic carbon is an sp3 carbon that is adjacent to a vinylic carbon.
None of the other hydrogens are vinylic.
Benzylic position allylic position propargylic position aryl aryl hydrogen.