In high dielectric ionizing solvents such as water dimethyl sulfoxide acetonitrile s n 1 and e1 products may be observed.
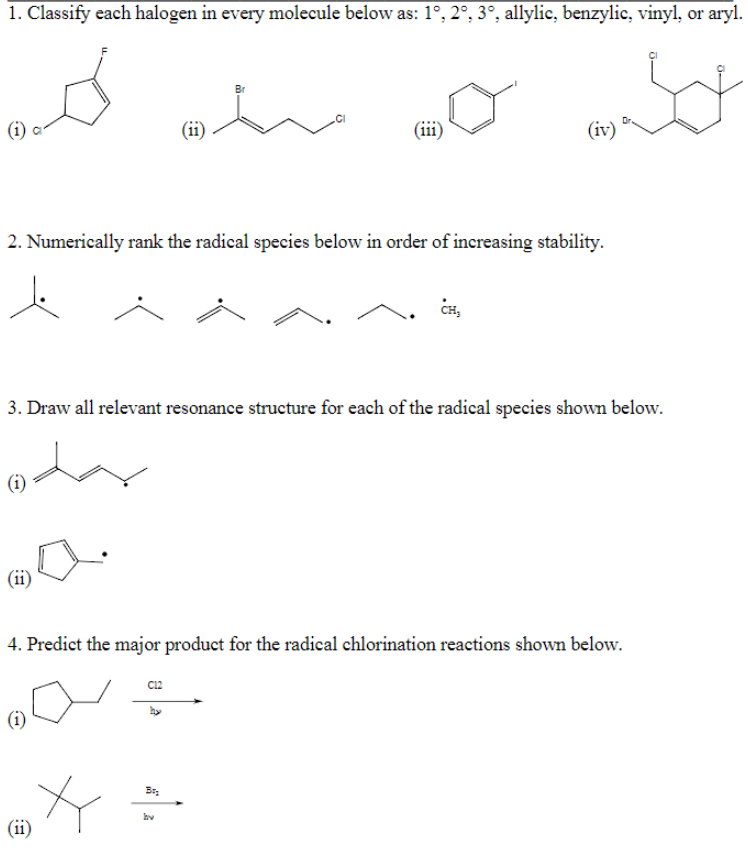
Allylic vinyl benzylic.
Hello friends main is video me aapko bataya hu common difference b w allylic benzylic vinylic aryl halides and alcohol tho dosto video ko acchi tarah dekhe acha lage tho like aur share kare.
An allyl derivative was first found within the garlic oil by theodor wertheim in 1844 he named it schwefelallyl.
Allyl group benzylic position propargylic position vinylic position alkene.
For 3º halides a very slow s n 2 substitution or if the nucleophile is moderately basic e2 elimination.
Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds.
Both show enhanced reactivity.
Rapid s n 2 substitution for 1º.
Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
Benzylic and allylic are related in terms of structure bond strength and reactivity.
The key difference between allylic and vinylic carbon is that allylic carbon is the carbon.
It would be kept mentioned that allyl is the latin word that is used for the garlic allium sativum.
Both allyl and vinyl groups have slightly similar structures with a small variation.
Allyl group consists of ch 2 methylene bridge and is attached with ch ch 2 vinyl group.
Benzylic groups are related to allyl groups.
The key difference between these two structural components is the number of carbon and hydrogen atoms.
The allylic positions are labeled with asterisks.
Other reactions that tend to occur with allylic compounds are allylic oxidations ene reactions and the tsuji trost reaction.
H h h h h vinyl allylic aryl benzylic n h h o h o oh nh2 o sh ch3 o oh h2 n oh oh o h o oh2 above 50 7 2 to 3 2 to 3 4 5 9 10 10 10 16 18 18 20 25 25 38 40 41 43 43 45 50.